Abstract
Introduction : Cytogenetic abnormalities by fluorescence in situ hybridization (FISH) are clinically relevant prognostic factors in MM. Data in transplant ineligible patients treated with bortezomib or lenalidomide in first-line therapy for high-risk (HiR) patients is limited. Careful analysis of cytogenetic subgroups in trials comparing different treatments remains an important goal. This sub-analysis evaluates the impact of cytogenetics on outcomes in transplant-ineligible patients with newly diagnosed MM (NDMM) treated with bortezomib-based induction (BORT) or lenalidomide-based (LEN) treatment.
Methods : In the GIMEMA-MM-03-05-trial, patients were randomized to bortezomib-melphalan-prednisone-thalidomide for 9 cycles followed by maintenance with bortezomib-thalidomide (VMPT-VT) vs VMP for 9 cycles, without maintenance. In the EMN01-trial, patients were randomized to melphalan-prednisone-lenalidomide (MPR) or cyclophosphamide-prednisone-lenalidomide (CPR) or lenalidomide plus low-dose dexamethasone (Rd) for 9 cycles, followed by maintenance with lenalidomide alone or plus prednisone continuously. Results of these studies have previously been reported (Palumbo A et al JCO 2010 and 2014; Magarotto V et al Blood 2016 127(9)).
Cytogenetics were assessed using FISH. Patients were categorized into cytogenetic risk groups according to International Myeloma Working Group criteria. HiR cytogenetics included del(17p), t(4;14), and t(14;16); all other patients were categorized as standard risk (StR).
Subgroup analyses were performed to determine the consistency of treatment effects of BOR vs LEN in the different subgroups using interaction terms between treatment and FISH, ISS, age, sex, Karnofsky PS and LDH. The different effect of BORT vs LEN in cytogenetic subgroups was confirmed by one sensitivity analysis where the follow-up of the BORT study was reduced to make the follow-up times similar; and by another sensitivity analysis with multiple imputation method for missing cytogenetic value.
Results : 902 of 1165 patients from the intent-to-treat population had available cytogenetic profiles, with 243 (27%) patients in the HiR group and 659 (73%) in the StR group. In the BORT vs LEN groups, median age was 71 vs 73 years (p<0.001), ISS3 20% vs 27% (P=0.65), HiR patients were 29% vs 26%, StR patients were 71% vs 74% (p=0.32) and the median follow-up was 72.3 and 63.6 months, respectively.
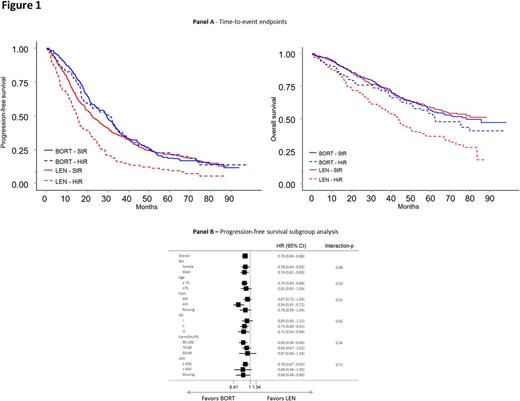
In the subgroup analysis, a significant difference was found in the cytogenetic subgroup with a superior advantage of BORT versus LEN in HiR group, whereas no significant difference was found between BORT and LEN in the other subgroups analyzed (ISS, age, sex, Karnofsky PS and LDH) (interaction-p=0.01) (Fig. 1 B).
BORT treatment resulted in a reduced risk of death or progression compared with LEN in patients with HiR. In HiR patients, median PFS was 30.8 with BORT compared with 14.8 months with LEN (HR: 0.54; 95% CI: 0.41-0.72); in StR, median PFS was 29.1 with BORT compared with 22.1 months with LEN (HR: 0.87; 95%; CI: 0.72-1.05) (Fig. 1 A).
Considering the standard of care VMP and Rd, in the HiR group (n=95) VMP resulted in a 48% reduced risk of death or progression compared with Rd (HR: 0.53; 95% CI: 0.34-0.83), whereas no significant difference in PFS was found in the StR group (n=273) (HR: 1.00; 95% CI: 0.75-1.33), interaction-p=0.02.
BORT treatment resulted in a reduced risk of death in patients with HiR cytogenetics: median OS was 62.4 months with BORT compared with 43.2 months with LEN (HR: 0.68; 95% CI: 0.47-0.96); in StR, median OS was 78.1 months with BORT and was not reached with LEN (HR: 1.06; 95% CI: 0.82-1.36), interaction-p=0.04 (Fig. 1 A).
In patients with del(17p) (n=131) median PFS was 18.0 vs 12.9 months for BORT vs LEN (HR: 0.71; 95% CI: 0.49-1.04), interaction-p=0.73. In patients with t(4;14) (n=118) median PFS was 31.5 vs 15.2 months for BORT vs LEN (HR: 0.41; 95% CI: 0.27-0.62) interaction-p=0.002. In patients with t(14;16) (n=31) median PFS was 36.2 vs 9.8 months for BORT vs LEN treated patients (HR: 0.34; 95% CI: 0.16-0.76), interaction-p=0.045.
Conclusions : BORT treatment resulted in a PFS and OS benefit vs LEN in patients with HiR cytogenetics. Treatment with VMP led to a significant reduction of the risk of death or progression vs Rd in HiR patients. These results support VMP induction as a standard treatment option for patients with NDMM who are ineligible for transplant with HiR cytogenetics.
Larocca: Celgene: Honoraria; Janssen: Honoraria; Bristol-Myers Squibb: Honoraria; Amgen: Honoraria. Offidani: celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Musto: Janssen: Honoraria; Celgene: Honoraria. Patriarca: MSD Italia: Honoraria; Janssen: Honoraria. Corradini: Gilead: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Roche: Honoraria; Celgene: Honoraria; Sanofi: Honoraria; Takeda: Honoraria; Novartis: Honoraria. Bosi: Amgen: Honoraria; Celgene: Honoraria; Janssen: Honoraria; Takeda: Membership on an entity's Board of Directors or advisory committees. Petrucci: Celgene: Honoraria; Janssen: Honoraria; Bristol-Myers Squibb: Honoraria; Takeda: Honoraria; Amgen: Honoraria. Boccadoro: Bristol-Myers Squibb: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Mundipharma: Research Funding; AbbVie: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal